Zoonotic Interfaces Under the Microscope
Salmonella spp. in Bats, Rodents, and Dogs in the Nan Region, Thailand.
By Dr. Alix NICOLAS, Veterinarian, as part of her final internship at the University of Liège, Belgium, in partnership with Kasetsart University, Thailand, for the Spillover Interface Project

During my final-year internship, I had the opportunity to take part in the BCOMING project, conducting research on zoonotic interfaces under the supervision of my thesis advisor, Dr. Mutien Marie Garigliany from the Department of Pathology at the Veterinary Faculty of Liège, and my internship supervisor, Dr. Johan Michaux, with the guidance of Dr. Pauline Van Leeuwen from the Conservation Genetics Laboratory at the Faculty of Biology in Liège. The main objective of this study was to determine the prevalence of Salmonella spp. in bats, rodents, and dogs in the Nan province of Thailand. With the recent COVID-19 pandemic, it has become evident that the emergence of new diseases is increasing. Previous epidemics, such as the Spanish flu in 1918, the avian flu in 2009-2010, and the various coronavirus outbreaks (SARS-CoV-1 in 2002-2003, MERS in 2012, and COVID-19—Jones et al., 2008; Smith et al., 2009), all share a common origin in animals.
The BCOMING project, funded by the EU and supported by international collaborations, aims to understand and prevent the emergence of new infectious diseases. In parallel, the Spillover Interface project shares similar goals, focusing on the link between biodiversity loss and the emergence of zoonotic diseases, while seeking to limit their transmission through conservation strategies and effective monitoring networks. These projects explore an urbanization gradient, from the least human-impacted ecosystems to the most urban, as well as a domestication gradient, including species ranging from wild to domestic. This approach allows us to study pathogen movement between species in various environments. The Spillover Interface Project, led by Dr. Serge Morand at Kasetsart University in Bangkok, Thailand, is a collaborative project of BCOMING. It extends the study by including a reforestation area, formerly agricultural land now returning to a more natural state, and by exploring the human-animal interface more deeply. The choice of Salmonella spp. as the study subject is relevant given the extensive knowledge of this pathogenic bacterium. Salmonellosis is a common infection in animals and humans, causing gastroenteritis in humans and being the third leading cause of foodborne death worldwide (Ferrari et al., 2019; Majowicz et al., 2010; Smith et al., 2014).
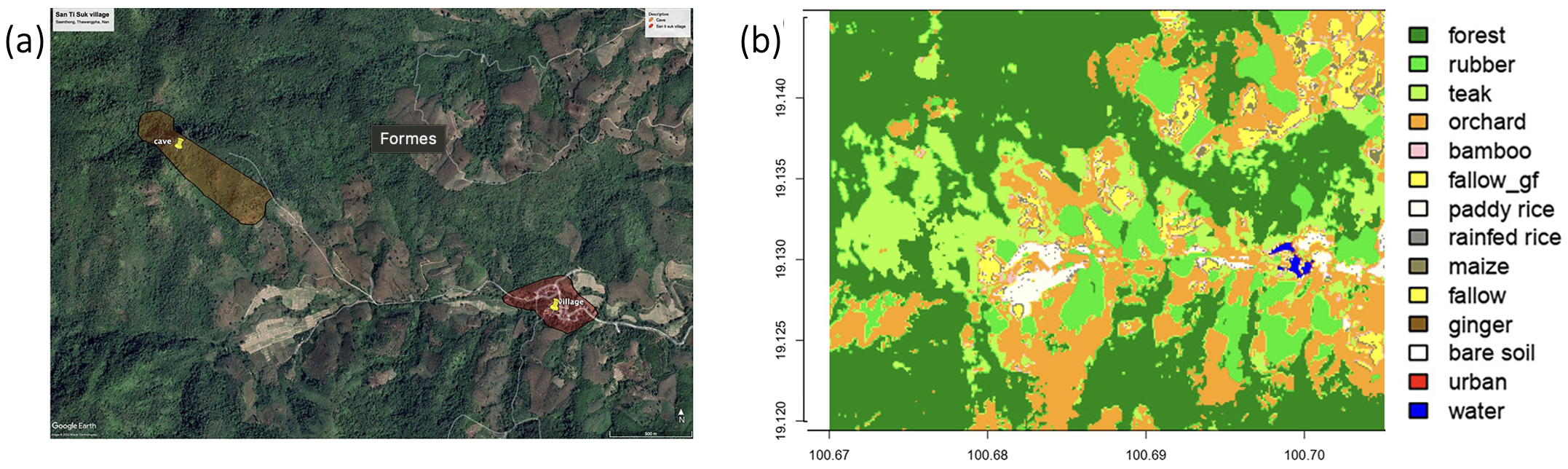
Figure 1: Excerpt from Thinphovong et al. (2024), Location of the study in the Saen Thong sub-district (Nan province, Thailand), specifically (a) in the highland area of the sub-district. A land use map shows the different land classes: multi-species forests, plantations (rubber, teak, bamboo plantations, orchards), fallow land (maize, paddy rice, ginger, etc.), urban infrastructure, the cave, and the village are indicated, (b) the highland part of the sub-district.
Our study uses samples collected in the Nan region of Thailand, near the Namtanburi
reserve. Figure 1 details the environment where the study was conducted. Our analyses were performed on rectal samples
from bats, small mammals, and dogs over several periods from February 2022 to February 2023. DNA was extracted using
standardized kits in the laboratory at Kasetsart University in Bangkok, Thailand, as shown in Photos 1 and 2. The
extracted DNA was then sent to Belgium to GECOLAB and the Pathology Laboratory at the Veterinary Department of the
University of Liège for analysis. A technique called quantitative PCR was used to specifically detect and
quantify Salmonella spp., focusing on strains dangerous to humans. This method amplifies a specific part of a gene
to detect the organism of interest. Finally, another quantitative PCR was performed to check that our samples contained
DNA and to what extent. This serves as a control to ensure that everything is functioning
properly.
Photos 1 and 2: Laboratory analysis of samples from the Spillover Interface project, Kasetsart University Laboratory, Bangkok (Thailand), taken on 29/11/23. Photo credit: Pauline Van Leeuwen.
Our results show that 12% of the samples were positive for Salmonella. Here is the breakdown by group:
- Bats (n=45): 20% positive, mainly in Scotophilus heathii, Hipposideros armiger, and Rhinolophus pusillus.
- Small mammals (n=48): None of the samples were positive.
- Dogs (n=23): 21.7% positive.
These results are somewhat biased by the low amount of DNA in the samples, suggesting a
possible underestimation of the true prevalence. Compared to expectations based on the literature (5% for bats, 7% for
dogs, and 15% for rodents—Dróżdż et al., 2020; Reyes et al., 2019; Ribas et al., 2016), our results are high for
bats and dogs. It is surprising that no small mammals tested positive.
Nevertheless, the study highlights the significant risk of Salmonella transmission in the Nan region. To reduce this risk, it is crucial to understand the movements of animals and, in particular, the interactions between wildlife, peri-urban animals, and domestic animals.
This study underscores the importance of monitoring zoonotic interfaces to understand and
attempt to prevent the emergence of new epidemics. The Nan region, with its diversity of habitats and species, offers a
unique opportunity to understand pathogen transmission dynamics. The next phase of this preliminary study includes a
microbiome analysis of the same samples, which will allow for a deeper understanding of these populations' dynamics, led
by Dr. Pauline Van Leeuwen. A publication is in progress, including the results of our two joint
studies.
Following this research, I had the honor of receiving the Tropical Veterinary Institute
Award from the University of Liège, a recognition that strengthens our commitment to continuing our efforts for public
and animal health. I would like to thank the teams from the BCOMING and Spillover Interface projects for this
opportunity and those who shared this great adventure with me.