The impact of trematodes on human and animal health in the world
Did you know? Food-borne trematodoses are zoonoses that can cause more than two million years of lost or disabled life worldwide each year.

People become infected when they eat raw fish, shellfish or vegetables that harbour the parasite larvae, which can cause severe liver and lung disease. The prevention and control of food-borne trematodiasis requires cross-sectoral collaboration at the interface between humans, animals and ecosystems. Trematodes, commonly known as flukes, are parasitic flatworms that affect both humans and animals worldwide. These parasites have complex life cycles involving multiple hosts. Within Bcoming, we seek to understand the impact of trematodes on human and animal health, including their transmission, the common diseases they cause and the socio-economic implications associated with these infections. Aquatic trematodes have complex life cycles (Fig. 1) that typically involve intermediate hosts, such as aquatic snails or fish, and definitive hosts, which can be humans or various animals.
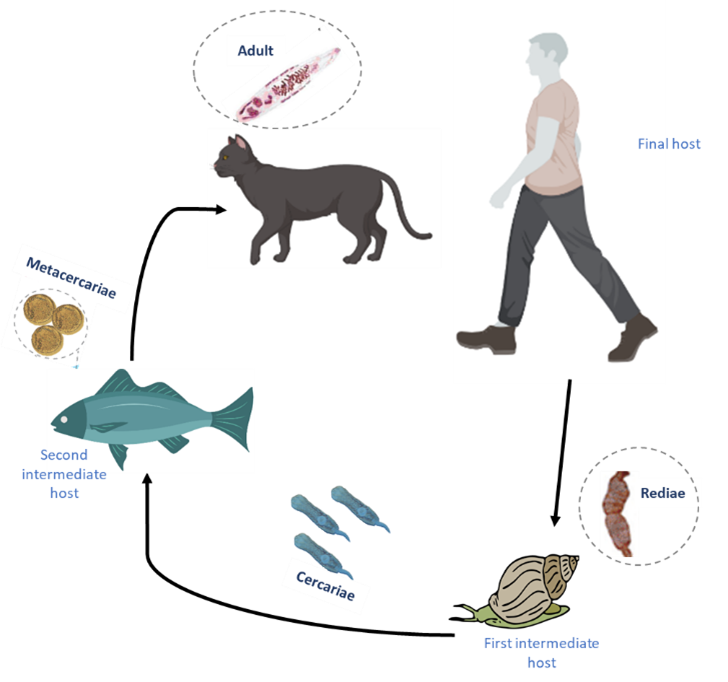
Figure. 1 Life cycle of trematode. The life cycle involves 1) a first intermediate host where rediae asexually produce cercariae, motile stages searching for 2) the second intermediate host, in which cercariae develop into the metacercaria stage that can live for months or years, waiting for the ingestion of the host by 3) the final host.
Trematode eggs are shed in the faeces of infected definitive hosts and hatch to infect snails, where they undergo several stages of development, which asexually produce infective larvae, the cercariae. Infected snails then release cercariae into the water, which can directly penetrate the skin of humans or animals upon contact with water, completing the life cycle, or infect a second intermediate host (i.e. fish or bivalve). Trematode infections can cause a variety of diseases in humans, resulting in significant morbidity and mortality in affected populations. Common diseases caused by trematodes include schistosomiasis (bilharzia), liver fluke infections, intestinal fluke infections and lung fluke infections. Schistosomiasis is one of the most common parasitic infections in tropical and subtropical regions. It is caused by blood flukes of the genus Schistosoma. Chronic infection can lead to enlargement of the liver and spleen, urinary tract complications, anaemia and even an increased risk of bladder cancer. Liver flukes such as Fasciola hepatica and Opisthorchis viverrini cause liver fluke infections. Infections can lead to hepatobiliary disease, including inflammation, fibrosis, bile duct obstruction and, in severe cases, cholangiocarcinoma (a type of bile duct cancer). Intestinal flukes, such as Fasciolopsis buski and Heterophyes heterophyes, infect the intestines. They can cause symptoms such as diarrhoea, abdominal pain, malnutrition and stunted growth in children. Pulmonary flukes, such as Paragonimus spp, infect the lungs and can cause respiratory symptoms such as cough, chest pain and bloody sputum.
Trematodes also pose significant health risks to animals, including livestock, pets and wildlife. Trematode infections in animals can lead to reduced productivity, weight loss, impaired growth, reproductive disorders and even death. The impact of trematode infections goes beyond health and affects the socio-economic well-being of communities and countries. Some of the most important socio-economic impacts include reduced productivity and healthcare costs. Trematode infections in humans and animals can lead to reduced productivity, affecting agricultural output, livestock production and economic growth, while the treatment of trematode infections requires medical interventions, drugs and diagnostic tests, resulting in significant healthcare costs for affected individuals and healthcare systems.
As part of Bcoming
We will sample the diversity of trematodes and hosts present in the environment along an urbanisation gradient to better understand the role of biodiversity in mitigating the impact of these parasites on local communities. We will visit Cambodia, Guinea, Côte d'Ivoire, and Guadeloupe. Applying a state-of-the-art approach using eDNA (https://theconversation.com/biodiversite-maladies-emergentes-epidemies-reveler-linvisible-grace-a-ladn-present-dans-lenvironnement-190389) as well as direct trematodes extractions from snails, we will measure the expected risk of infection from the environment as well as the realised risk in local populations.
In July, we are planning a field trip across Cambodia to collect some biodiversity and eDNA samples. The team will consist of Dr. Marine Combe and Claudia Bommarito, Ms Chloé Lefevbre and Prof. Rudy E. Gozlan from the French National Research Institute for Sustainable Development and the Institute of Evolutionary Sciences of Montpellier, France. In Phnom Pehn, our team will be hosted by our colleagues of the Pasteur Institute of Cambogia, Dr. Julia Guillebaud, Neavuthea Keng, Veasna Duong and Sreyly Kao and during the field trip our team will be accompanied by two representants of the staff of the Inland Fisheries Research and Development Institute (IFReDI) of Fisheries Administration, Mr. Thach Phanara and Mr. Hok Seiha. The field sampling will include six different geographical zones 1) Battambang 2) Siem Reap 3) Krong Stung Treng 4) Mondulkiri 5) Phnom Penh 6) Takeo, characterized by high or low levels of urbanization (Fig. 2).
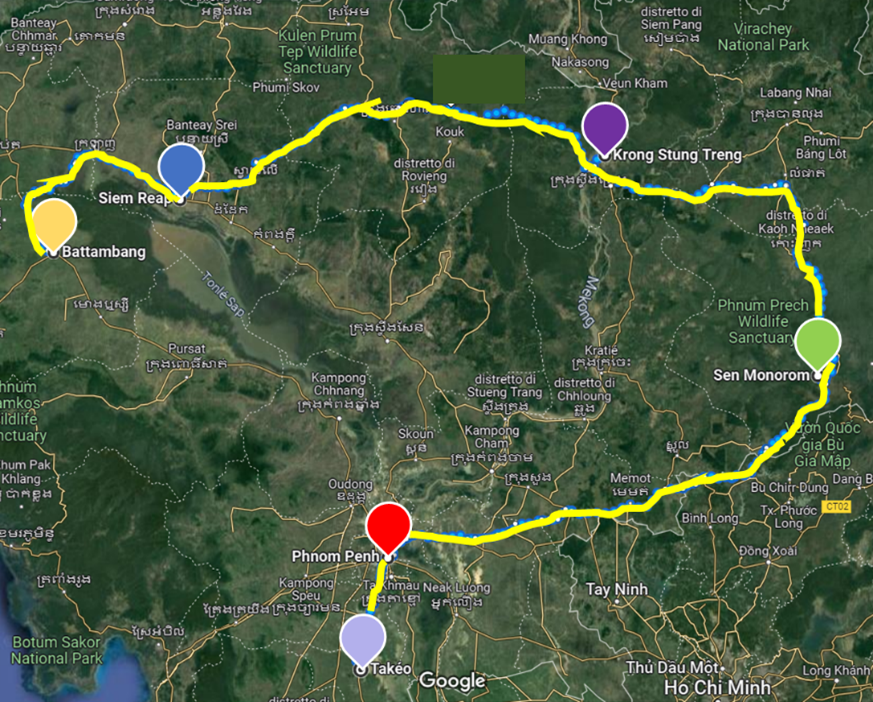
Figure. 2 Map of the six sampling zones in Cambogia 1) Battambang (in yellow), 2) Siem Reap (in blue) 3) Krong Stung Treng (in purple) 4) Sen Monorom/Mundulkuri (in green) 5) Phnom Penh (in red) 6) Takeo (in violet).In each zone, sampling will be carried out at five different sites covering a gradient of urbanisation and habitat diversity (ponds, lakes, river, tributaries). At each site, water samples will be collected for eDNA and snails, and abiotic and biotic parameters will be recorded. The snail samples will be processed in the laboratory and the trematode species will be identified morphologically and molecularly. The eDNA samples will be processed and analysed by collaborating companies, Spygen for trematodes and gastropods and Nature Metrics for fish and microbiome. Parasites, host presence and abundance, and their diversity will be analysed using models with urbanisation and climate change drivers as independent variables.
This sampling will be a wonderful adventure for our team, not only as an opportunity to obtain excellent scientific data on overlooked host and parasite communities, but also to enrich our naturalistic knowledge of tropical habitats and our cultural knowledge, thanks to the collaboration with our colleagues in Cambodia. The study will enable the development of new intercontinental collaborations and help to build a more robust and interdisciplinary network between ecologists, stakeholders and government, which is becoming increasingly urgent in a world subject to anthropisation and climate change that threatens the health of people, animals and ecosystems.